1. Make sure the sample is representative and contains a visible lesion.
2. The sample should include the lesion as well as normal tissue at the outer edge.
3. Make the incision with a sharp knife or scalpel. Do not use scissors, as they will crush and deform the sample.
4. Use forceps carefully to avoid deforming the sample.
5. The sample should not be more than 0.5 to 1 cm in thickness so that adequate penetration of the formalin is possible and the sample is fixed.
6. The size of the sample is not that important if the fixative is used in sufficient quantity. In general, the sample should not be more than 3 cm square.
7. To be sure that you will have the necessary sample to submit, the sample should include the lesion as well as normal tissue at the outer edge. A sample should include, for example: tonsils, thyroid – parathyroid, lungs, papillary muscles of the left ventricle, bronchial lymph nodes, liver, kidneys, spleen, pancreas, urinary bladder, stomach, intestines, mesenteric lymph nodes.
Pathoanatomy and Pathohistology
The mission of the Pathology Department (PD) is to provide timely, accurate anatomopathological, histopathological, and immunohistochemical results. Upon request from veterinarians and when the magnitude of the problem indicates such, the pathologist is available for consultation. In every activity, we are committed to the recognition, diagnosis, and control of animal diseases.
PD is an integral part of the diagnostic protocol at LUV. Dead animals (sheep, goats, and poultry) arrive for necropsy, and after examination by the pathologist, the tissues are sent to other LUV departments for further testing. PD is practically the most important part of the general diagnosis at LUV, and deals with all aspects of laboratory diagnosis. This section deals specifically with pathological cases of importance and interest.
Pathological findings of diagnostic interest
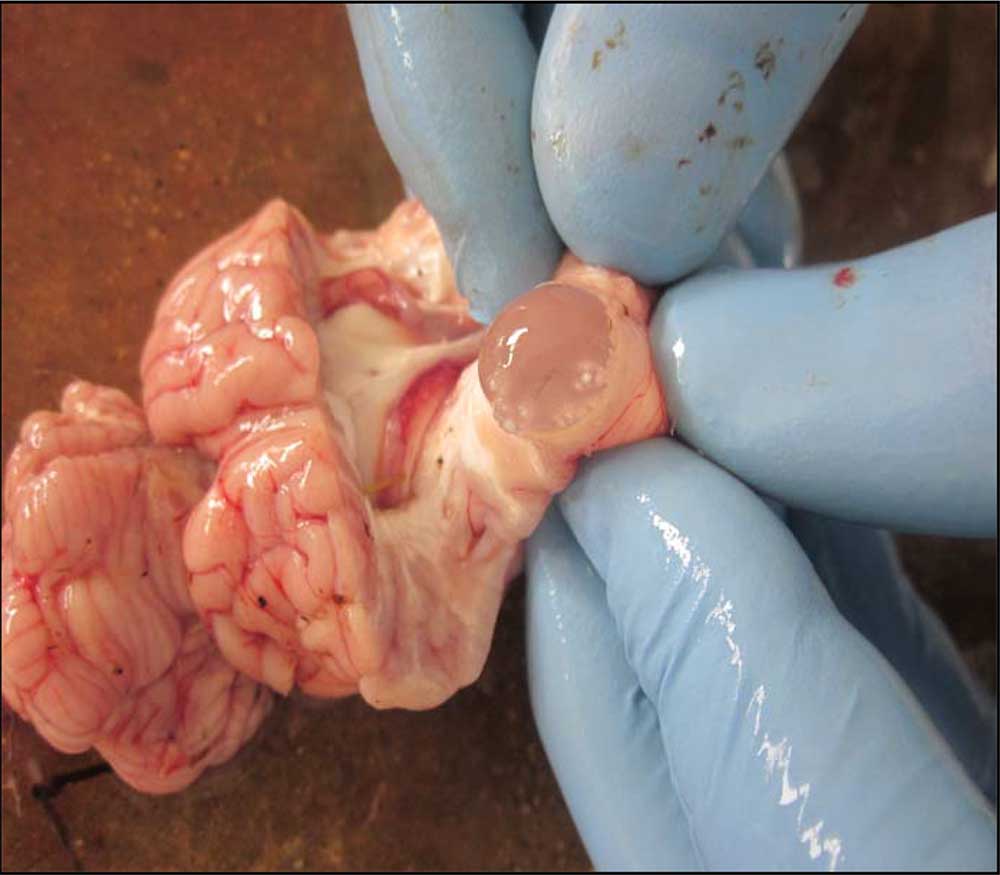
Coenurosis
The thin-walled cyst (coenurus cerebralis) in the cerebral hemisphere represents the larval form of the dog (and other canid) tapeworm Tenia multiceps.

Cisticercosis
Cysts (cisticercus tenuicollis) in the liver represent the larval form of the dog (and other canid) tapeworm, Tenia hydatigena.

Sarcocystosis
Parasite of the genus Sarcocystis within the muscles of the goat’s esophagus.

Listeriosis
Microabscess and perivascular infiltration in the sheep’s marrow indicate the neutral form of listeriosis. The bacteria Listeria monocytogenes was confirmed by isolation.
Sending samples to the Pathology Laboratory
As described below, in order to have optimal anatomopathological and histopathological results, it is necessary to collect and send samples regularly.
Samples that can be sent to the Pathology Sector are: dead animals, heads for rabies testing, fresh organs and samples in formalin.
Dead animals must be submitted for examination immediately after death. Do not freeze carcasses for anatomopathological examination!
Fresh organ samples must be placed in plastic bags, refrigerated and transported in ice packs. Samples for histopathology must be placed in formalin
Taking and Storing Samples for Histopathological Examination.
Sampling
1. Use 10% phosphate buffered formalin.
Prepare one liter of 10% phosphate buffered formalin.
Formaldehyde solution (37-40%) 100 ml
Distilled water 900 ml
Monobasic Sodium Phosphate 4.0 g
Dibasic Sodium Phosphate 6.5 g
2. Place 10% formalin in a container that has a mouth width nearly equal to its diameter. This is necessary to allow easy removal of the tissues after they have been fixed. .
3. When placing specimens in 10% formalin, provide 10 volumes of fixative for each volume of tissue. This will ensure that all tissue surfaces come into contact with the fixative.
4. Tissues for histopathological evaluation should never be frozen. Freezing and thawing disrupts cells and causes artifacts.
Sample storage
Rabies
SP diagnoses rabies and also helps control rabies and sylvatic rabies.
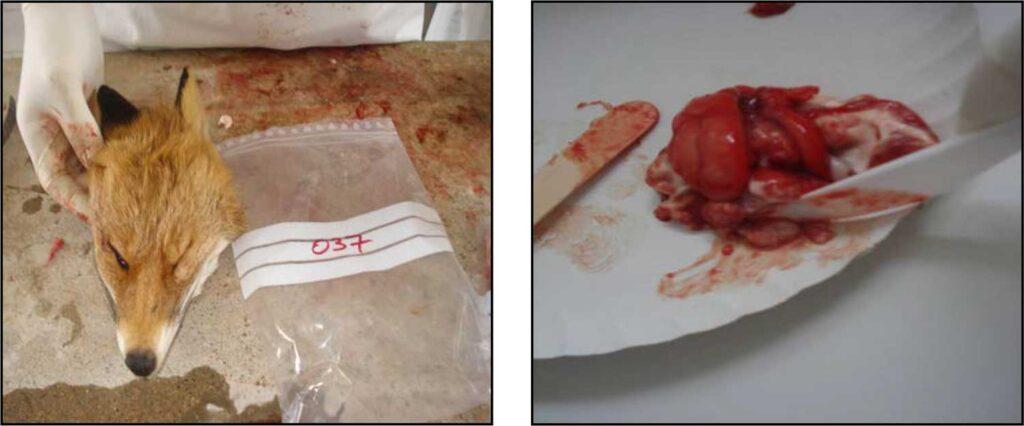
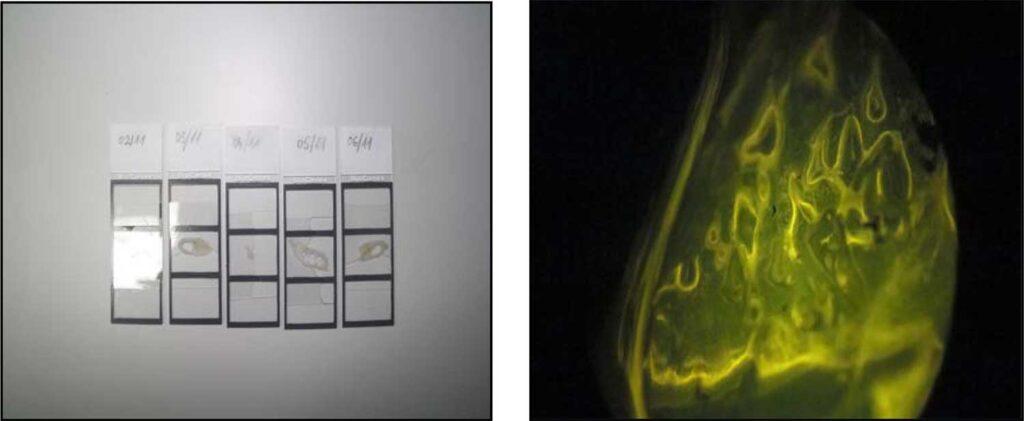
The success of oral vaccination in foxes is monitored through the vaccine marker in the bones.
Some types of samples

 Shqip
Shqip Serbian
Serbian